INTRODUCTION:Flumatinib mesylate is a derivative of imatinib and has higher selectivity and potency toward BCR::ABL1 kinase compared with imatinib. Flumatinib was approved patients with newly diagnosed CML-CP in China. We analysed the efficiency and safety of flumatinib in CML patients resistant or intolerant to Imatinib in the real word.
METHODS:164 adult CML-CP patientsrecieved flumatinib as 2nd line therapy after imatinib were collected from 18 centers in china. The primary outcome was to demonstrate the probabilities of responses including complete hematologic response (CHR), cytogenetic response, and molecular response (MR) after the later line of flumatinib. The secondary outcome was to assess adverse events (AEs). The diagnosis and response evaluation were defined according to the European Leukemian Net 2020 recommendations. The side effect were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 4.0.
Results: 164 patients with CML-CP with a median age of 51 (range: 17-87) years were included, 95(57.9%) males. The medium interval from imatinib to flumatinib was 13.2(1-185) months. The median dosage of flumatinib was 600 mg/day. 127 patients had ELTS information with 84 (66.1%), 30 (23.6%), 13 (10.2%) patients in low-, medium-, and high risk groups, respectively. ABL mutations were detected in 144 patients before initiating flumatinib, 5 (3.5%) patients had ABL mutations with 1 patient each showing E279K, E459K, M351T mutation; and 2 patients had F317L mutation. I 56 (34.1%) patients had optimal response but intolerant to imatinib, whereas 43 (26.2%), 65 (39.5%) patients had suboptimal or failure response to imatinib.
The median duration of flumatinib treatment was 10.9 (2-25.46) months. Discontinuation of flumatinib treatment was observed in 17 (11%) patients, with a median treatment duration of 8.8 (2-25.46) months.
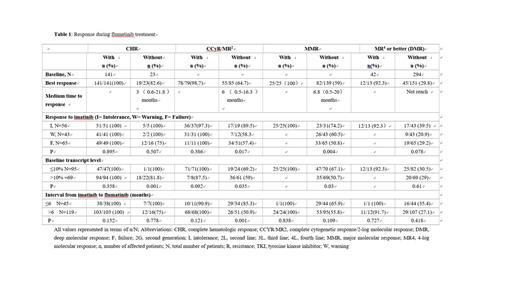
The probabilities of CHR, CCyR/MR 2, MMR, and MR4 or better at baseline and after flumatinib therapy have been represented in Table 1. The patients without evaluable data were considered without response. The rate of CHR, CCyR/MR 2, MMR and MR4 or better were 86% (141/164), 48.2% (79/164), 15.2% (25/164) and 7.9% (13/164) at baseline increased to 97.6% (160/164). 81.1% (133/164), 65.2% (107/164), 34.8% (57/164) respectively during flumatinib treatment.
The probabilities of response from CHR to MR after flumatinib therapy were analyzed based on clinical parameters. The gender, age, and ELTS score at diagnosis had no influence on the response to flumatinib, whereas the response to imatinib, the interval from imatinib to flumatinib, transcript levels at baseline were associated with the response to flumatinib. Patients who were intolerant to imatinib had higher rates achieving CCyR/MR 2, MMR, and DMR when compared with those with warning or failure response to imatinib. The median time to CCyR/MR 2, MMR, and DMR was 3.7 months, 4.7 months, and 15 months in intolerant patients; 3.8 months, 7.5 months, and not reached in warning patients; 7.1 months, 8.7 months, and not reached in failure patients, respectively. The patients with transcript ≤10% at baseline showed a higher probability of achieving CCyR/MR 2 and MMR as compared with those with transcript >10% at baseline, while the probabilities to achieve DMR were similar in both groups. The patients switched to flumatinib within 6 months and without CCyR/MR2 at baseline had a higher probability and shorter medium time of achieving CCyR (85.3% vs 51%, 3.3 vs 9.9months). The patient with E279Km and resistant to imatinib achieved MMR with flumatinib treatment. 1 patient with F317Lm achieved MR4. The patient with M351Tm received flumatinib for 4.5 months and with decreasing transcript level, 1 patient with E459Km received flumatinib for 4 months and maintained CHR with stable BCR::ABL transcript levels.
27 patients had hematological toxicities during exposure to Flumatinib. Most nonhematological AEs were grade ≤2, and only 2 patients showed grade ≥3 diarrhea. The major flumatinib-related nonhematological AEs included gastrointestinal AEs ( n = 29), rash ( n = 9), and creatinine elevation ( n =7).
conclusion:This retrospective study had suggested promising effects of flumatinib, which showed induced high rates of CCyR and MMR or DMR in patients resistant or intolerant to imatinib. The incidence of AEs during flumatinib treatment was tolerable.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal